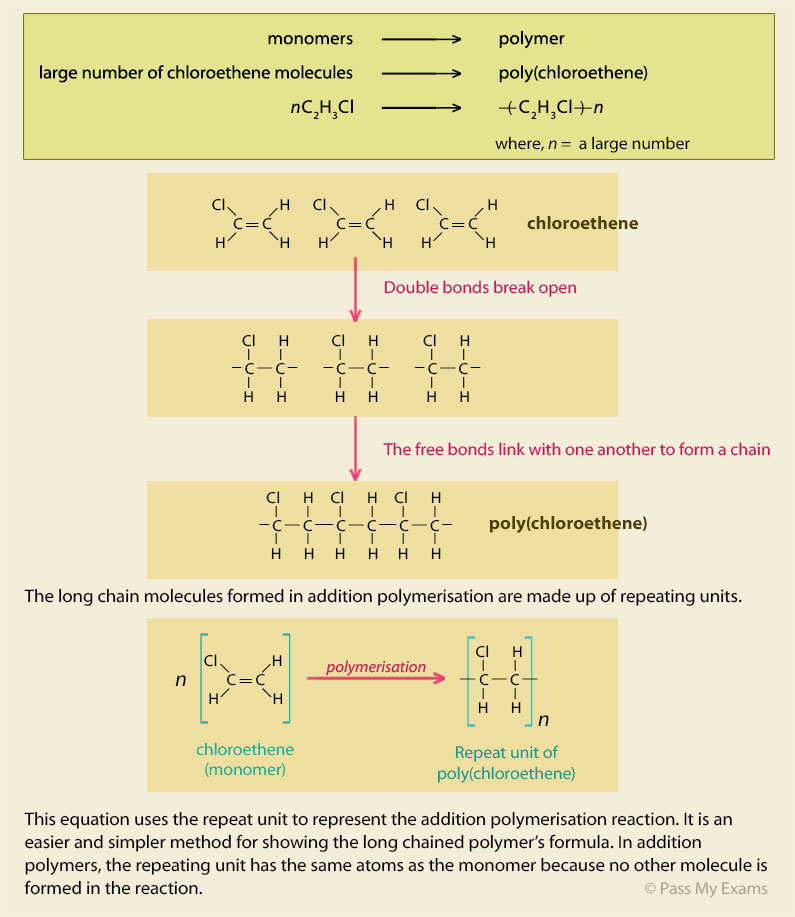
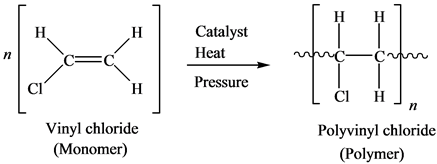Polymerization Of Vinyl Chloride Equation
Goodrich plant near louisville kentucky were diagnosed with liver angiosarcoma also known as hemangiosarcoma a.
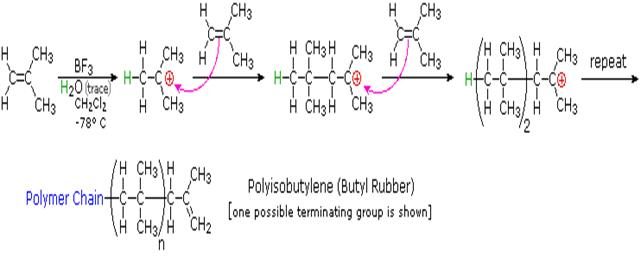
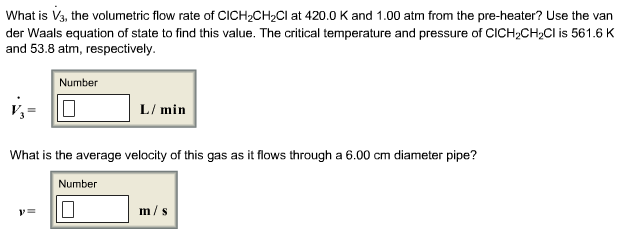
Polymerization of vinyl chloride equation. Vc vc αd 1 vc β βd 2 and vc d 3 were used to study the reactivities of the hydrogen atoms in the polymerization and the β hydrogen atoms contributed to the chain transfer. Polymerization of vinyl chloride vc was studied. Poly vinyl chloride is also known as poly vinyl or vinyl as abbreviated known as pvc. In the early 1970s the carcinogenicity of vinyl chloride usually called vinyl chloride monomer or vcm was linked to cancers in workers in the polyvinyl chloride industry.
Specifically workers in polymerization section of a b f. Being a very well known memb r of the family of vinyl polymers. Pvc ranks as the second most important polymer after ethylene. Pvc is the widely third most large using synthetic polymer in world followed by polyethylene polypropene.
Polyvinylchloride is the polymer formed from the polymerization of the monomer vinyl chloride. The equation representing the synthesis of polyvinylchloride from vinyl chloride is as follows. The case of vinyl chloride suspension polymerization. Polyvinyl chloride pvc chemical formula.
The term polyvinyl chloride or pvc indicates homopo1ymers of vinyl chloride and incorrectly copolymers containing amow1ts of vinyl idene chloride vinyl acetage ethylene propylene or acrylates. Refer to table 11 2. Importance of radical transfer in precipitation polymerization. This polymerisation reaction proceeds by a free radical mechanism.
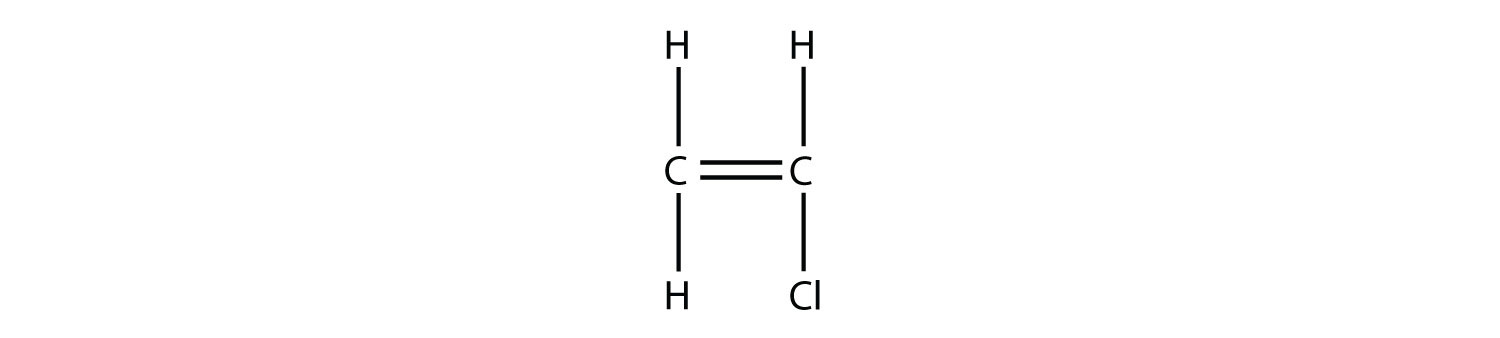
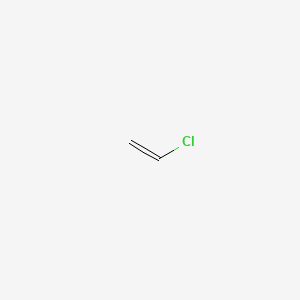
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc. Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer. Polyvinyl chloride is a white brittle solid. Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.
Polyvinyl chloride is a white rigid quite brittle solid. Chemical and physical methods were used to observe irregular structures such as branching. About 13 billion kilograms are produced annually. Pvc vinyl chloride is an organohalogen compound that has important industrial applications.
Additives are used to modify the properties of polyvinyl chloride to make it more useful. Pvc is used in the manufacture of numerous products including packaging films and water pipes. Write an equation representing the synthesis of polyvinyl chloride from vinyl chloride.