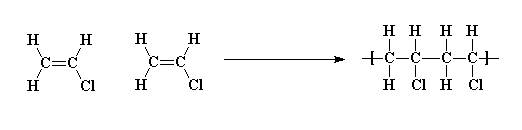Poly Vinyl Chloride Dipole
Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
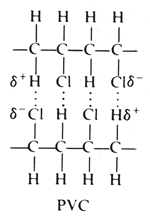
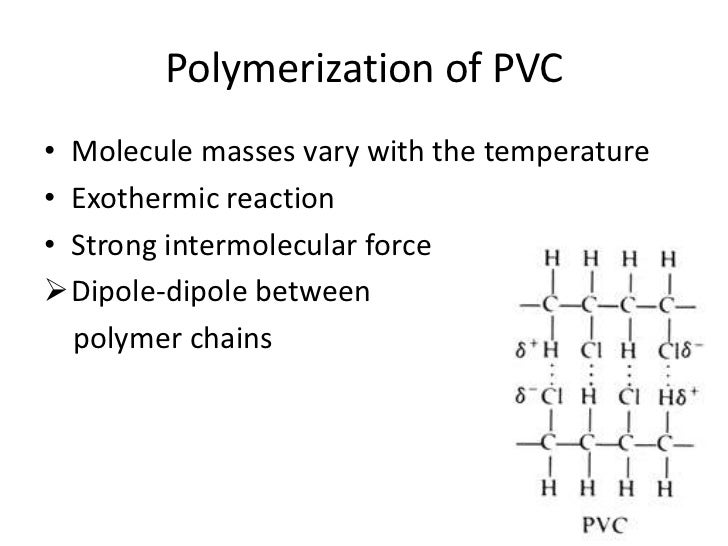
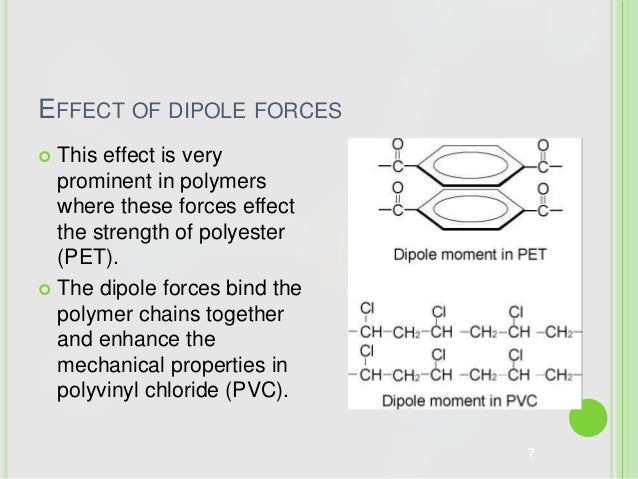
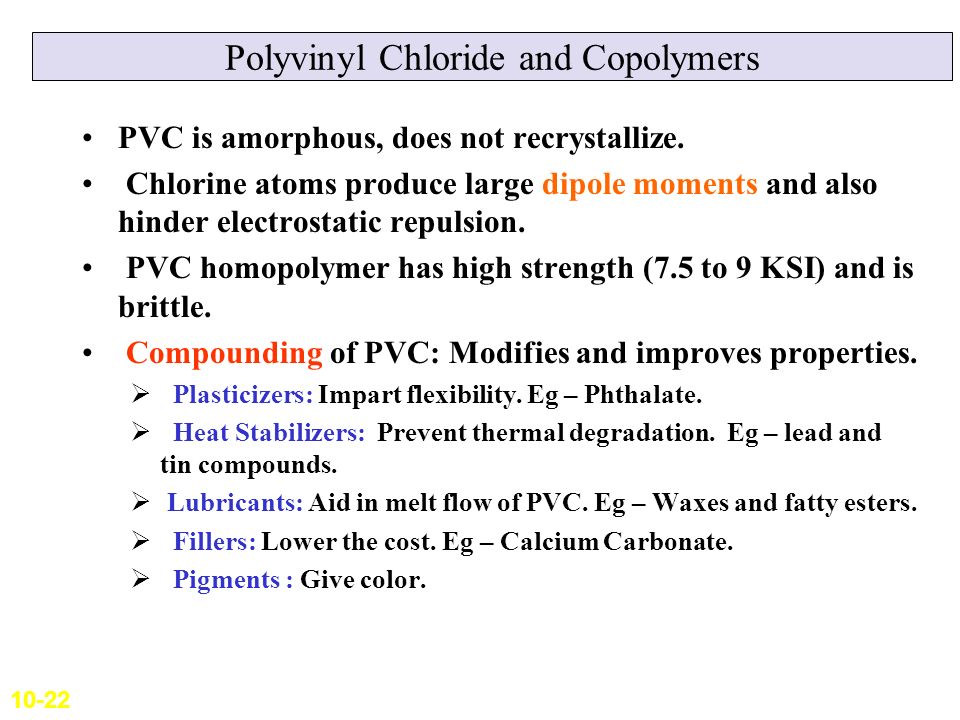
Poly vinyl chloride dipole. Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer. Pvc is a polymer of vinyl chloride. These considerable intermolecular attractions between polymer chains make pvc a fairly strong material. Polyvinyl chloride is a white rigid quite brittle solid.
Rigid sometimes abbreviated as rpvc and flexible. Pvc comes in two basic forms. Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene. Cruz et al.
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc. This polymerisation reaction proceeds by a free radical mechanism. Pvc vinyl chloride is an organohalogen compound that has important industrial applications. Additives are used to modify the properties of polyvinyl chloride to make it more useful.
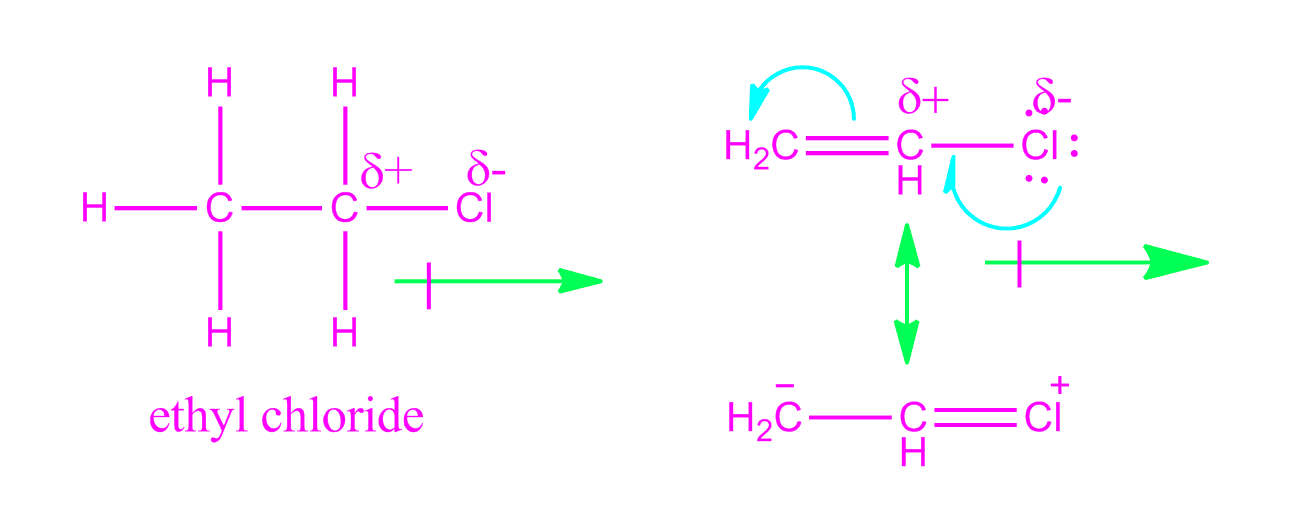
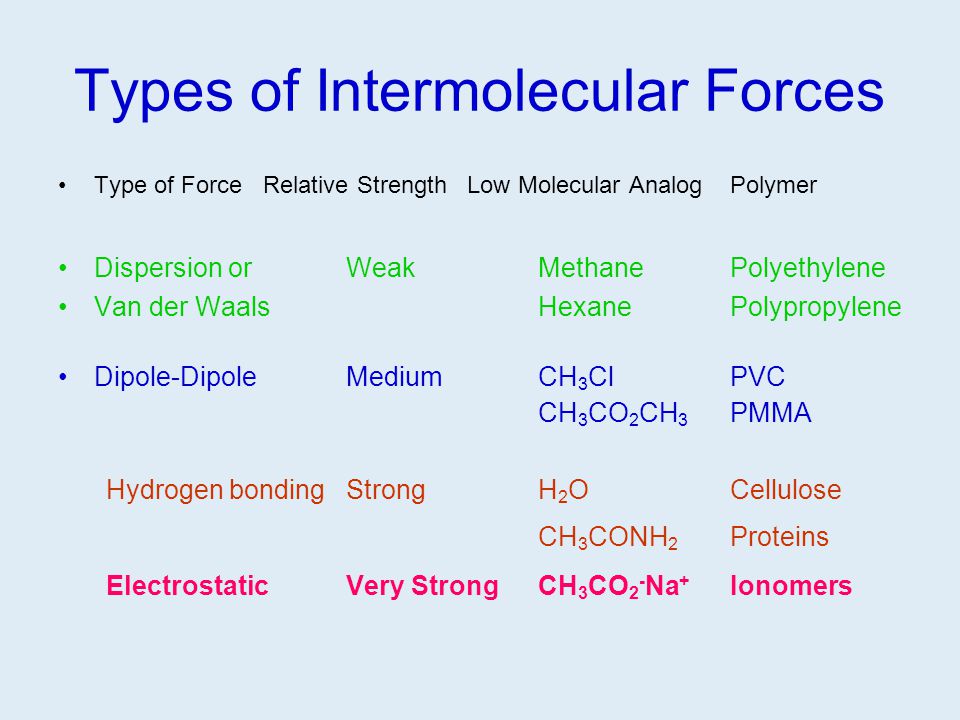
It is tempting to say that the molecules line up as in the diagram below. For instance eknoian and his coworkers prepared copolymers of oxazolidinone monomers with acrylonitrile polyvinyl chloride polyvinyl alcohol polyvinyl acetate and styrene through an emulsion. The most important forces in pvc are dipole induced dipole attractions. Poly vinyl chloride dipole dipole interaction.
It looks as if are dipole dipole forces between adjacent molecules but the cl atoms are large and they stick out from the chain in random directions. About 40 million tons of pvc are produced each year. Ahmed a and yousif e. Prud homme 11 poly carbonate range of polyesters.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.