Poly Ethylene Co Vinyl Acetate Melting Point

Poly ethylene co vinyl acetate vinyl acetate 25 wt.
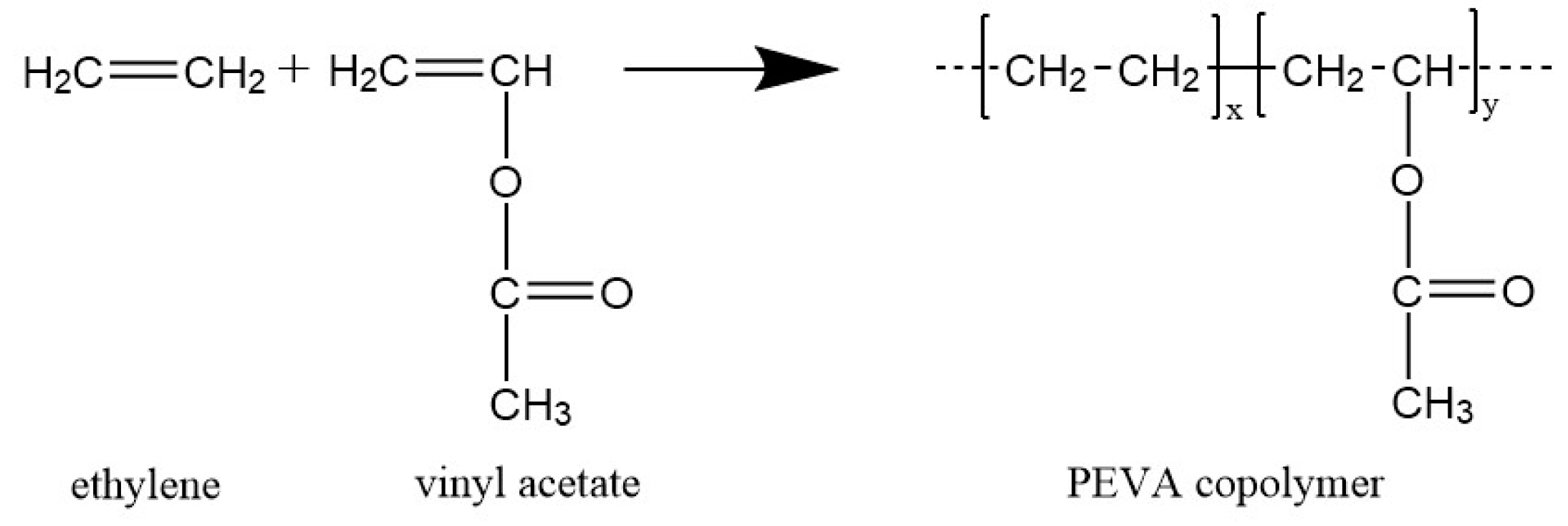
Poly ethylene co vinyl acetate melting point. Product name property description. 87 c 189 f boiling point no data available. Poly ethylene co vinyl acetate 4 product results match criteria. Ethylene vinyl acetate eva also known as poly ethylene vinyl acetate peva is the copolymer of ethylene and vinyl acetate the weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene.
Fabrication characterization and properties of poly ethylene co vinyl acetate composite thin films doped with piezoelectric nanofillers. Pubchem substance id 24867357. Product description 340502. Poly ethylene co vinyl acetate vinyl acetate 25 wt.
Poly ethylene co vinyl acetate product number. Poly ethylene co vinyl acetate 4 product results match criteria. 2f 1 207 dunhua n. Poly vinyl acetate was discovered in germany in 1912 by fritz klatte.
Polysciences asia pacific inc. Asia pacific sales and distribution. Melt index 19 g 10 min 190 c. Melt index 57 g 10 min 190 c 2 16kg contains 200 800 ppm bht as inhibitor 437220.
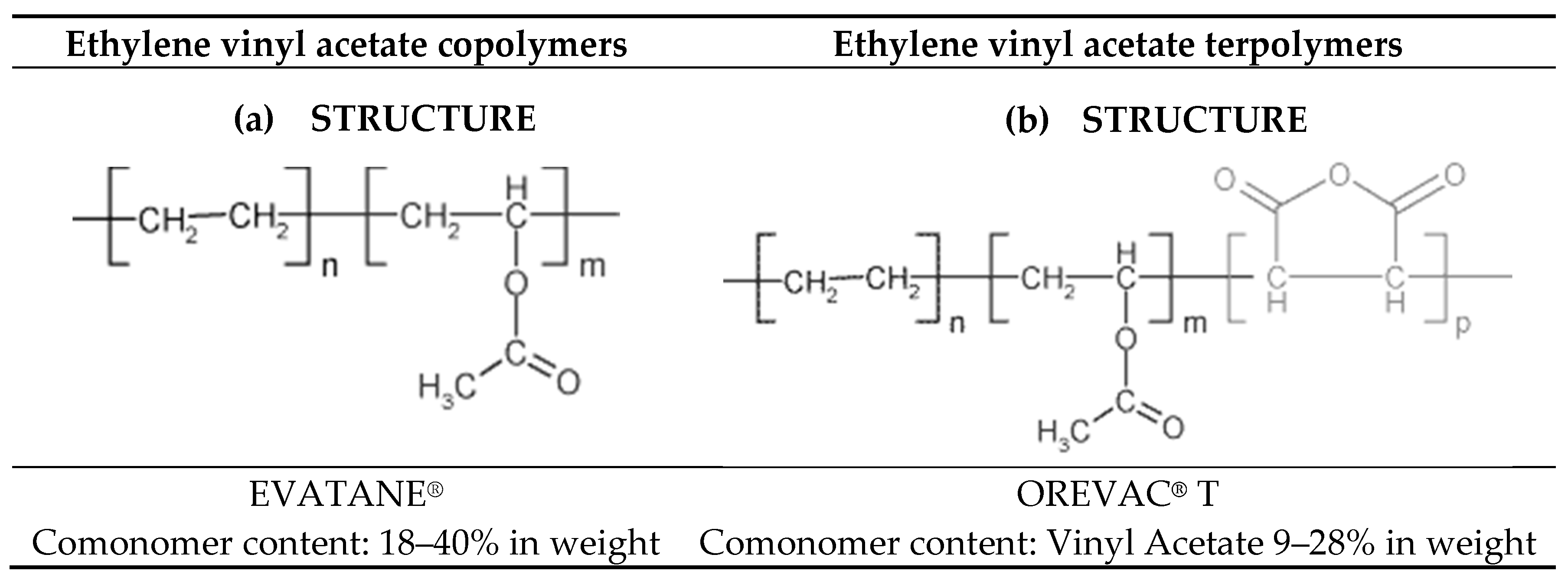
Taipei taiwan 10595 886 2 8712 0600 886 2 8712 2677 fax info polysciences tw. Aldrich 437239 page 4 of 7. Microstructures of poly ethylene co vinyl acetate eva are dependent on the distribution of va units as well as the va content simple analytical method to determine microstructure of eva was established using melting points of eva and correlationship with those of wax and its ester. Poly ethylene co vinyl acetate vinyl acetate 40 wt.
There are three different types of eva copolymer which differ in the vinyl acetate va content and the way the materials are used. Melting point density additive chemical composition melt index hardness. Giulia mariotti and lorenzo vannozzi. The material melting point was obtained from the second heating run.
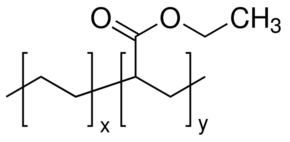
Linear formula ch 2 ch 2 m ch 2 ch ococh 3 n. Melting point freezing point melting point range. 3 the monomer vinyl acetate was first produced on an industrial scale by the addition of acetic acid to acetylene with a mercury i salt 4 but it is now primarily made by palladium catalyzed oxidative addition of acetic acid to ethylene.